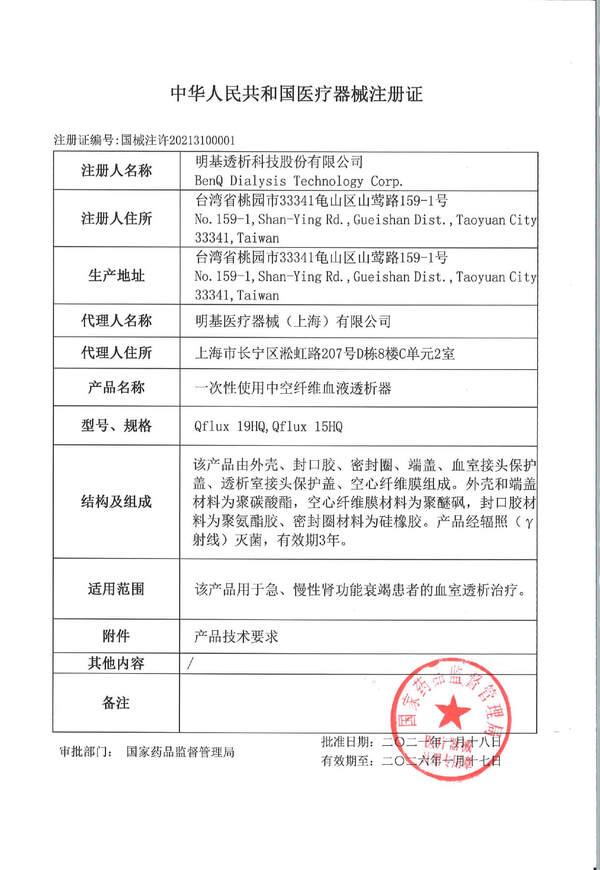
.jpg)
Quality Management System
Adhering to the international standards of GMP & ISO 13485, from raw materials, finished products, storage environment to clean room production, all are strictly checked and controlled to provide stable, safe and effective products.
International Standard
GMP & ISO 13485
International Quality Assurance Certification
Air conditioning and water system monitoring
BenQ Continuous Improvement Project CIP
Safety and effectiveness
196 product inspections
Inspection Items

Material specification measurement, hollow fiber membrane analysis, cleanliness inspection.
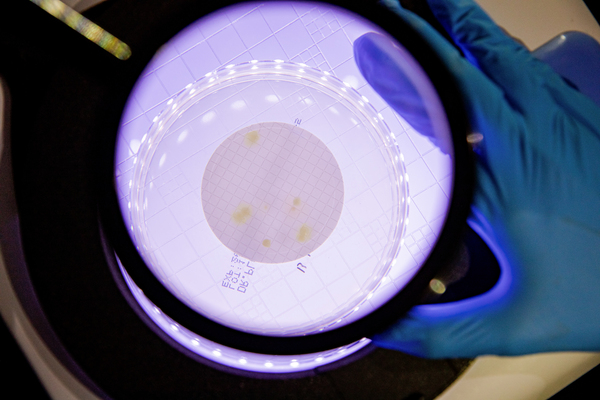
Regularly airborne microbe inspection, process water microbe and endotoxin inspection.

Laser sintering inspection, section inspection, AOI automatic optical inspection,package assembly integrity test , leak testing.

Finished product performance testing, endotoxin testing, bioburden testing, sterilization dose monitoring.
Milestones
-
2014/10
BenQ Dialysis Techonology Corp. Founded
-
2014/12
Agreement with Medica For Agency And Technology Transfer
-
2016/07
Factory and Assembly Line Ready.Pilot Run Finished
-
2017/03
ISO 13485 And GMP(Taiwan) Passed
-
2017/12
BenQ Qflux Dialyzer TFDA Registration
-
2018/03
Launch in Taiwan
-
2018/05
Launch in Thailand
-
2018/08
Merge K2 International Medical
-
2018/11
KFDA Inspection Passed.
Launch in Korea
-
2020/02
Launch in Vietnam
-
2020/06
Merge Golden Spirit Co., Ltd, and start to sell dialysate
-
2021/02
Launch in Philippines
-
2021/03
Launch in China
-
2021/06
Launch in Indonesia
-
2022/02
Launch in Malaysia


.png)