.jpg)
Manufacturing
The only dialyzer manufacturer in Taiwan, utilizing professional automatic manufacturing process, which passed GMP & ISO 13485 quality certification, provides safe and effective products with stable quality.
GMP & ISO13485
The only made-in-Taiwan hemodialyzer
Strictly monitor process cleanliness
The in-plant laboratory checks product quality immediately
Factory Introduction
.jpg)
The centrally monitored 10,000 rating clean room (C area) implements the air-conditioning validation plan based on ISO 14644, regularly performs airborne bacteria testing, and provides a clean environment to ensure production quality.
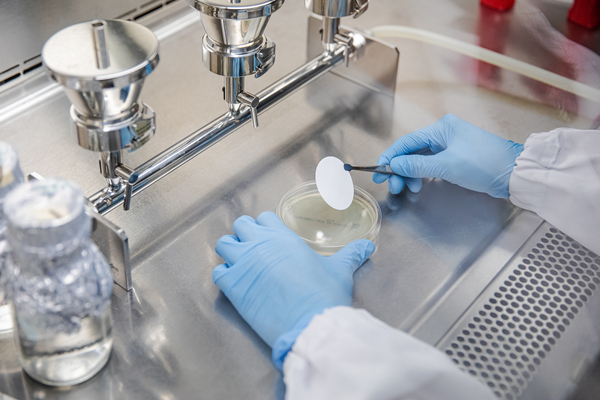
A testing laboratory that meets the requirements of ISO 17025 which can instantly check product quality.

The storage environment is controlled by temperature and humidity to maintain the good quality of raw materials and finished products.

Two Pass R.O.+UF is used to perform monthly microbiological and endotoxin testing to ensure that the pure water meets ANSI / AAMI / ISO 23500-1 international standards, and maintain the quality of pure water required for the production of medical equipment.

Each dialyzer is automatically inspected by the AOI system to ensure perfect assembly quality.

Each dialyzer will pass a leak test, and an automatic robotic arm will remove defective products to ensure the completeness of the hollow fiber membrane and dialyzer.

The production line is transferred by automatic arms to reduce personnel contact and microbial contamination.

Product labeling and packaging are automatically completed to avoid human error.
.png)
.png)
.jpg)
